
SL Paper 2
Titanium is a transition metal.
TiCl4 reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the atom.
State the full electron configuration of the 2+ ion.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Distinguish ultraviolet light from visible light in terms of wavelength and energy.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
State the block of the periodic table in which magnesium is located.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
__ Mg3N2 (s) + __ H2O (l) → __ Mg(OH)2 (s) + __ NH3 (aq)
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
PCl5(g) and Cl2(g) were placed in a sealed flask and allowed to reach equilibrium at 200 °C. The enthalpy change, ΔH, for the decomposition of PCl5(g) is positive.
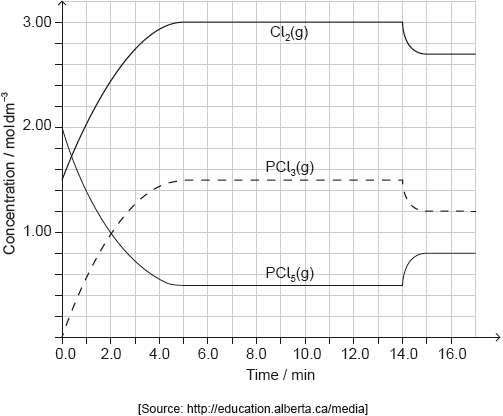
Deduce the equilibrium constant expression, Kc, for the decomposition of PCl5(g).
Deduce, giving a reason, the factor responsible for establishing the new equilibrium after 14 minutes.
Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.
Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State the formula of the salt formed when butanoic acid reacts with ethylamine.
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
In acidic solution, hydrogen peroxide, H2O2, will oxidize Fe2+.
Fe2+ (aq) → Fe3+ (aq) + e−
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
Write the half-equation for the reduction of hydrogen peroxide to water in acidic solution.
Deduce a balanced equation for the oxidation of Fe2+ by acidified hydrogen peroxide.
Bonds can be formed in many ways.
The landing module for the Apollo mission used rocket fuel made from a mixture of hydrazine, N2H4, and dinitrogen tetraoxide, N2O4.
N2H4(l) + N2O4(l) → 3N2(g) + 4H2O(g)
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
Determine the oxidation state of nitrogen in the two reactants.
Deduce, giving a reason, which species is the reducing agent.
Deduce the Lewis (electron dot) structures of ozone.
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Explain the polarity of PCl3.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
This question is about compounds of sodium.
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Describe the structure and bonding in solid sodium oxide.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00 g of sodium oxide produces 5.50 g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (c)(i).
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
Product A contains a carbon–carbon double bond. State the type of reactions that compounds containing this bond are likely to undergo.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ. Predict, giving a reason, which product is the most stable.
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
Sulfur trioxide is produced from sulfur dioxide.
2SO2 (g) + O2 (g) 2SO3 (g) ΔH = −196 kJ mol−1
The reaction between sulfur dioxide and oxygen can be carried out at different temperatures.
Nitric acid, HNO3, is another strong Brønsted–Lowry acid. Its conjugate base is the nitrate ion, NO3−
Outline, giving a reason, the effect of a catalyst on a reaction.
On the axes, sketch Maxwell–Boltzmann energy distribution curves for the reacting species at two temperatures T1 and T2, where T2 > T1.
Explain the effect of increasing temperature on the yield of SO3.
State the product formed from the reaction of SO3 with water.
State the meaning of a strong Brønsted–Lowry acid.
Draw the Lewis structure of NO3−.
Explain the electron domain geometry of NO3−.
Automobile air bags inflate by a rapid decomposition reaction. One typical compound used is guanidinium nitrate, C(NH2)3NO3, which decomposes very rapidly to form nitrogen, water vapour and carbon.
Deduce the equation for the decomposition of guanidinium nitrate.
Calculate the total number of moles of gas produced from the decomposition of 10.0 g of guanidinium nitrate.
Calculate the pressure, in kPa, of this gas in a 10.0 dm3 air bag at 127°C, assuming no gas escapes.
Suggest why water vapour deviates significantly from ideal behaviour when the gases are cooled, while nitrogen does not.
Another airbag reactant produces nitrogen gas and sodium.
Suggest, including an equation, why the products of this reactant present a safety hazard.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
State the electron configuration of the Ca2+ ion.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
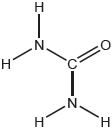
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.
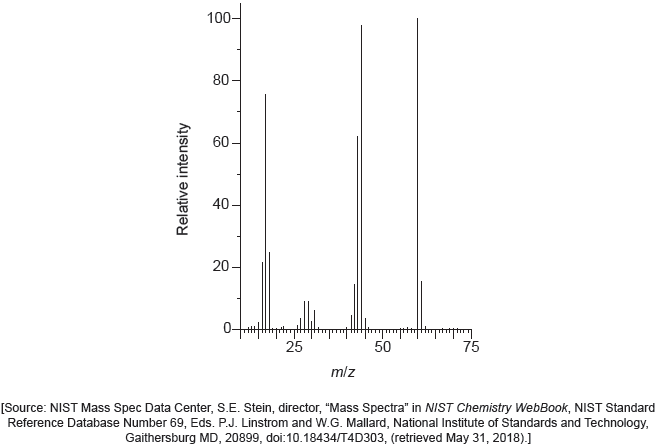
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
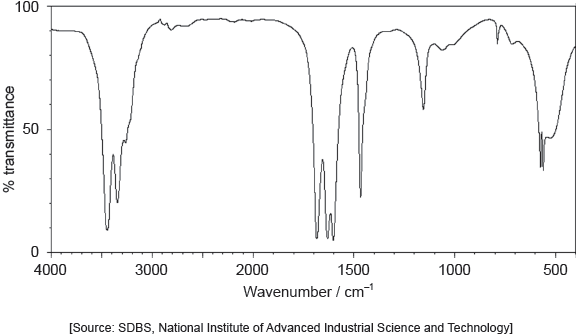
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Benzoic acid, C6H5COOH, is another derivative of benzene.
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
The concentration of a solution of a weak acid, such as ethanedioic acid, can be determined
by titration with a standard solution of sodium hydroxide, NaOH (aq).
Distinguish between a weak acid and a strong acid.
Weak acid:
Strong acid:
Suggest why it is more convenient to express acidity using the pH scale instead of using the concentration of hydrogen ions.
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(ii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iii) Determine the percentage purity of the hydrated ethanedioic acid sample.
The Lewis (electron dot) structure of the ethanedioate ion is shown below.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.
Ammonia, NH3, is industrially important for the manufacture of fertilizers, explosives and plastics.
Ammonia is produced by the Haber–Bosch process which involves the equilibrium:
N2 (g) + 3 H2 (g) 2 NH3 (g)
The effect of temperature on the position of equilibrium depends on the enthalpy change of the reaction.
Ammonia is soluble in water and forms an alkaline solution:
NH3 (g) + H2O (l) NH4+ (aq) + HO– (aq)
Draw arrows in the boxes to represent the electron configuration of a nitrogen atom.
Draw the Lewis (electron dot) structure of the ammonia molecule.
Deduce the expression for the equilibrium constant, Kc, for this equation.
Explain why an increase in pressure shifts the position of equilibrium towards the products and how this affects the value of the equilibrium constant, Kc.
State how the use of a catalyst affects the position of the equilibrium.
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
Calculate the enthalpy change, ΔH⦵, for the Haber–Bosch process, in kJ, using the following data.
.
Suggest why the values obtained in (d)(i) and (d)(ii) differ.
State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
Determine the concentration, in mol dm–3, of the solution formed when 900.0 dm3 of NH3 (g) at 300.0 K and 100.0 kPa, is dissolved in water to form 2.00 dm3 of solution. Use sections 1 and 2 of the data booklet.
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
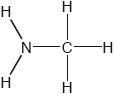
Two hydrides of nitrogen are ammonia and hydrazine, N2H4. One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
N2H4(aq) + O2(aq) → N2(g) + 2H2O(l)
The concentration of dissolved oxygen in a sample of water is 8.0 × 10−3 gdm−3.
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
Ammonia reacts reversibly with water.
NH3(g) + H2O(l) NH4+(aq) + OH−(aq)
Explain the effect of adding H+(aq) ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Hydrazine has been used as a rocket fuel. The propulsion reaction occurs in several stages but the overall reaction is:
N2H4(l) → N2(g) + 2H2(g)
Suggest why this fuel is suitable for use at high altitudes.
Determine the enthalpy change of reaction, ΔH, in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
N2H4(g) → N2(g) + 2H2(g)
The standard enthalpy of formation of N2H4(l) is +50.6 kJmol−1. Calculate the enthalpy of vaporization, ΔHvap, of hydrazine in kJmol−1.
N2H4(l) → N2H4(g)
(If you did not get an answer to (f), use −85 kJ but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from 1000 dm3 of the sample.
Calculate the volume, in dm3, of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = 24.8 dm3 at SATP.)
Iron (II) sulfide reacts with hydrochloric acid to form hydrogen sulfide, H2S.
In aqueous solution, hydrogen sulfide acts as an acid.
Draw the Lewis (electron dot) structure of hydrogen sulfide.
Predict the shape of the hydrogen sulfide molecule.
State the formula of its conjugate base.
Saturated aqueous hydrogen sulfide has a concentration of 0.10 mol dm−3 and a pH of 4.0. Demonstrate whether it is a strong or weak acid.
Calculate the hydroxide ion concentration in saturated aqueous hydrogen sulfide.
A gaseous sample of nitrogen, contaminated only with hydrogen sulfide, was reacted with excess sodium hydroxide solution at constant temperature. The volume of the gas changed from 550 cm3 to 525 cm3.
Determine the mole percentage of hydrogen sulfide in the sample, stating one assumption you made.
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, , for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
Nickel catalyses the conversion of propanone to propan-2-ol.
Outline how a catalyst increases the rate of reaction.
Explain why an increase in temperature increases the rate of reaction.
Discuss, referring to intermolecular forces present, the relative volatility of propanone and propan-2-ol.
The diagram shows an unlabelled voltaic cell for the reaction
Label the diagram with the species in the equation.
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
Describe the bonding in metals.
Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the addition of another metal to nickel.
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain the decrease in radius from Na to Na+.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) 2SO3 (g)
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Some physical properties of molecular substances result from the different types of forces between their molecules.
Explain why the hydrides of group 16 elements (H2O, H2S, H2Se and H2Te) are polar molecules.
The graph shows the boiling points of the hydrides of group 16 elements.

Explain the increase in the boiling point from H2S to H2Te.
Lewis structures show electron domains and are used to predict molecular geometry.
Deduce the electron domain geometry and the molecular geometry for the NH2− ion.
Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce the molecular geometry of each species.
Predict with a reason, whether the molecule PF3 is polar or non-polar.
Bromine can form the bromate(V) ion, BrO3−.
State the electron configuration of a bromine atom.
Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. Use boxes to represent orbitals and arrows to represent electrons.
Draw the Lewis (electron dot) structure for BrO3− that obeys the octet rule.
Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Magnesium is a reactive metal often found in alloys.
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
Magnesium can be produced by the electrolysis of molten magnesium chloride.
Write the half-equation for the formation of magnesium.
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
State the name of Compound A, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Identify the strongest force between the molecules of Compound B.
Draw the structural formula of the alkene required.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Describe the bonding in this type of solid.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.
Properties of elements and their compounds can be related to the position of the elements in the periodic table.
Explain the decrease in atomic radius from Na to Cl.
Explain why the radius of the sodium ion, Na+, is smaller than the radius of the oxide ion, O2−.
State a physical property of sodium oxide.
Both vinegar (a dilute aqueous solution of ethanoic acid) and bleach are used as cleaning agents.
Bleach reacts with ammonia, also used as a cleaning agent, to produce the poisonous compound chloramine, NH2Cl.
Outline why ethanoic acid is classified as a weak acid.
A solution of bleach can be made by reacting chlorine gas with a sodium hydroxide solution.
Cl2 (g) + 2NaOH (aq) NaOCl (aq) + NaCl (aq) + H2O (l)
Suggest, with reference to Le Châtelier’s principle, why it is dangerous to mix vinegar and bleach together as cleaners.
Draw a Lewis (electron dot) structure of chloramine.
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
The following shows some compounds which can be made from ethene, C2H4.
ethene (C2H4) → C2H5Cl → C2H6O → C2H4O
State the type of reaction which converts ethene into C2H5Cl.
Write an equation for the reaction of C2H5Cl with aqueous sodium hydroxide to produce a C2H6O compound, showing structural formulas.
Write an equation for the complete combustion of the organic product in (b).
Determine the enthalpy of combustion of the organic product in (b), in kJ mol−1, using data from section 11 of the data booklet.
State the reagents and conditions for the conversion of the compound C2H6O, produced in (b), into C2H4O.
Explain why the compound C2H6O, produced in (b), has a higher boiling point than compound C2H4O, produced in d(i).
Ethene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
Carbonated water is produced when carbon dioxide is dissolved in water under pressure.
The following equilibria are established.
Carbon dioxide acts as a weak acid.
Soda water has sodium hydrogencarbonate, NaHCO3, dissolved in the carbonated water.
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
When a bottle of carbonated water is opened, these equilibria are disturbed.
State, giving a reason, how a decrease in pressure affects the position of Equilibrium (1).
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
100.0 cm3 of soda water contains 3.0 × 10−2 g NaHCO3.
Calculate the concentration of NaHCO3 in mol dm−3.
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
Carbon forms many compounds.
C60 and diamond are allotropes of carbon.
But-2-ene reacts with hydrogen bromide.
Chlorine reacts with methane.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Outline one difference between the bonding of carbon atoms in C60 and diamond.
State two features showing that propane and butane are members of the same homologous series.
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
Draw the full structural formula of but-2-ene.
Write the equation for the reaction between but-2-ene and hydrogen bromide.
State the type of reaction.
Suggest two differences in the 1H NMR of but-2-ene and the organic product from (d)(ii).
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
Draw and label an enthalpy level diagram for this reaction.